Hi, I'm Raji Kooner. For the last 25 years, I've been providing specialist urology services in Sydney at a variety of hospitals, including St. Vincent's Private Hospital, Mater Hospital, and East Sydney Private Hospital.
I'm delighted today to talk about a new, innovative treatment for moderate to severe BPH called the iTind. iTind stands for “temporary implantable device.” It utilizes the unique properties of nitinol wire—a nickel and titanium shape memory alloy—making it ideal for use in endoscopic procedures.
This device reshapes the prostate and is typically used for prostates under 60 cc, although it has also been used in glands larger than 80 cc. Notably, the iTind procedure does not cause sexual dysfunction, does not involve permanent implants, and does not require injection into the prostate.
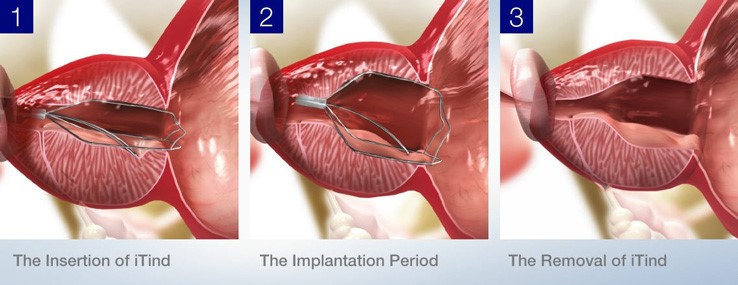
The iTind device is inserted via a cystoscope and anchored just below the bladder neck. It stays in place for 5 to 7 days, expanding gradually to compress the prostate tissue. This compression creates three channels—at the 5, 7, and 12 o'clock positions—improving urine flow.
After 5 to 7 days, the device is removed via a sheath. The removal process is quick and typically done under light sedation in an outpatient setting. The overall procedure is minimally invasive and in Australia is usually performed in a day surgery unit.
Prior to the procedure, standard assessments such as urine flow testing, ultrasound, urine culture, and flexible cystoscopy are performed. Patients are also reviewed by an anaesthetist, who discusses expectations and plans post-procedure care including pain relief and antispasmodics.
Removal of the device involves gently pulling it out using a snare through a catheter, and it typically reverts to its original shape once removed. The entire experience is designed to be low-impact for the patient while providing meaningful symptom relief.
iTind represents a significant step forward in minimally invasive urological care, and I believe it’s an excellent alternative for patients seeking to avoid long-term medication or more invasive surgery.
Thank you for watching.
Further information:
What is iTind?
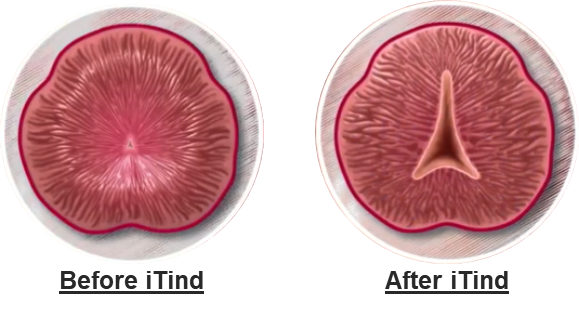
The iTind procedure reshapes the anatomy of the prostatic urethra, gently creating a wider opening for urine to flow freely, without burning or cutting out tissue, and without leaving behind a permanent implant. The treatment is straightforward, does not require overnight hospitalisation, and has none of the side effects associated with prescription medication. Additionally, one of the main advantages of the iTind procedure is the preservation of sexual and ejaculatory functions2, 3, 4.
iTind: A New Minimally Invasive Treatment for BPH
Dr Kooner is pleased to be one of the first Urologists in Australia to perform the iTind procedure to treat men with the symptoms of enlarged prostate. The iTind procedure is a new minimally invasive therapy for benign prostatic hyperplasia (BPH), offering an alternative to surgery, drugs, and permanent implants.
Dr Kooner has been implanting iTind since September 2022. He says, "The iTind is an important addition to our treatment portfolio, offering a less invasive treatment option with no major side effects."1
The iTind procedure reshapes the anatomy of the prostatic urethra, gently creating a wider opening for urine to flow freely, without burning or cutting out tissue, and without leaving behind a permanent implant. The treatment is straightforward, does not require overnight hospitalisation, and has none of the side effects associated with prescription medication. Additionally, one of the main advantages of the iTind procedure is the preservation of sexual and ejaculatory functions2, 3, 4.
Having performed the iTind procedure on many patients to date, Dr Kooner says it is a promising procedure providing relief for BPH patients.
iTind Information Brochures
iTind Patient Flyer
iTind Patient Information - What You Need To Know
iTind Mechanism of Action and Clinical Results